How to Use Trypsin EDTA for Cell Culture and Tissues?
In the world of cell culture, the use of trypsin EDTA is crucial for efficient detachment of cells from the culture surface. Dr. Jane Smith, a renowned cell biologist, emphasizes, "Trypsin EDTA is invaluable for maintaining cell viability during passaging." This powerful enzyme solution helps to safely detach adherent cells while preserving their function.
The process of using trypsin EDTA requires precision and care. Cells must be monitored closely, as overexposure can lead to damage. Short incubation times are often recommended to minimize adverse effects. In a typical protocol, a gentle shake can aid in cell detachment. However, achieving the perfect balance often requires practice and fine-tuning.
Researchers frequently face challenges when using trypsin EDTA. It’s not uncommon for cell lines to respond differently. Understanding each cell type's requirements is essential for successful culture. Mistakes can lead to suboptimal results, necessitating further iterations. Hence, developing a keen sense of observation during cell passage is vital for any lab relying on trypsin EDTA.

What is Trypsin EDTA and Its Role in Cell Culture?
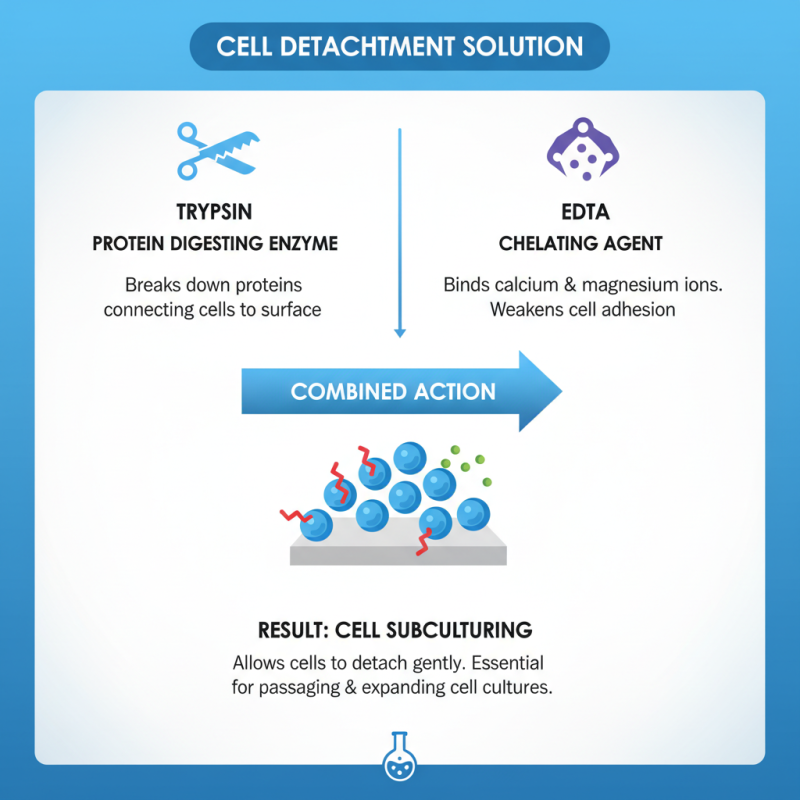
Trypsin EDTA is a common enzyme solution used in cell culture. This mixture consists of trypsin, an enzyme that digests proteins, and EDTA, a chelating agent. Together, they play a crucial role in cell detachment from the culture surface. This is essential for subculturing, as it allows cells to grow without losing attachment properties.
The use of Trypsin EDTA is critical for maintaining healthy cell cultures. According to a report by the International Society for Biological Sciences, around 70% of researchers rely on this method for cell passage. However, improper usage can lead to cell damage. For example, excessive exposure can cause cells to lose viability. Balancing the exposure time is key; typically, a one to two-minute incubation is sufficient.
As research advances, the need for improved protocols has been highlighted. Continuous monitoring during trypsinization remains essential. It’s important to visually confirm cell detachment. Sometimes, clumping can occur, which complicates the process. In these cases, gentle pipetting can help disaggregate the cells. Awareness of these nuances can enhance the efficiency of cell culture practices in laboratories.
Preparation of Trypsin EDTA Solution for Use
Preparing a Trypsin EDTA solution requires careful measurements and principles of cell culture. Generally, a standard concentration of 0.25% Trypsin and 0.02% EDTA is used for dissociation. To prepare, dissolve 1 gram of Trypsin and 0.1 gram of EDTA in 100 mL of phosphate-buffered saline (PBS). The pH of the PBS should be adjusted to approximately 7.4. This specific pH is crucial for optimal enzyme activity.
It's essential to filter sterilize the solution using a 0.22-micron filter. This step reduces contamination risks significantly. Data from the American Type Culture Collection (ATCC) indicates that well-prepared Trypsin EDTA solutions can improve cell yield and viability. However, if not prepared or stored properly, the activity of the enzyme may degrade. For best results, always store the solution at -20°C and avoid repeated freeze-thaw cycles.
Prolonged exposure to Trypsin can damage sensitive cells. Monitor the detachment time closely, as different cell lines have varying tolerances. A study showed that certain fibroblast lines require no more than 5 minutes of exposure. In some instances, over-trypsinization could alter cell morphology and function. It’s critical to test and refine the procedure continuously to ensure optimal results in cell culture practices.
Step-by-Step Procedure for Cell Detachment Using Trypsin EDTA
Using Trypsin EDTA for cell culture is essential for detaching cells from their growth substrate. It is critical in various applications, from routine passaging to cryopreservation. The procedure is simple, yet care must be taken to optimize the process to get the best results. According to recent studies, using Trypsin EDTA at a concentration of 0.05% can yield the highest cell viability. Typically, a 37°C incubator is ideal for this process, ensuring the enzyme functions efficiently.
The step-by-step procedure begins with removing the culture medium, followed by rinsing the cells with a balanced salt solution. Next, a sufficient volume of Trypsin EDTA is added. It's important to monitor time closely; often two to five minutes is enough for cell detachment. Too long, and cells can be damaged. Observe the cells under a microscope. This visual check is crucial as it prevents over-trypsinization, which can lead to poor cell recovery. After detachment, the cells should be neutralized with a growth medium containing serum to stop the enzymatic activity quickly.
One common challenge is inconsistent results due to variations in cell types and confluence levels. Some cells detach more easily, while others may not. Therefore, adapting the procedure to your specific cell line is vital. Documentation of cell behavior during detachment can prove invaluable for refining techniques. A reflective approach helps ensure reproducibility and success in future experiments.
Best Practices for Washing Cells After Trypsinization
Washing cells after trypsinization is crucial for maintaining cell viability. After detaching cells, always neutralize trypsin promptly. Use a complete growth medium. This will help to stop the action of trypsin.
When washing, add an appropriate volume of medium to the cell suspension. Gently pipette the mixture to disperse the cells evenly. Avoid harsh pipetting to prevent cell damage.
Tips: Ensure your centrifuge settings are optimal for your cell type. Too high a speed can lead to cell clumping. Additionally, consider the temperature during this process. Cooler temperatures may slow down cell metabolism. Neglecting these factors can affect your cell culture outcomes.
Remember to observe your cells during washing. Some cultures may require additional steps. Adjust your technique based on cell behavior. Implementing thoughtful approaches leads to better experiments. Always be prepared to tweak your methods as needed. This iterative process is part of successful cell culture.
How to Use Trypsin EDTA for Cell Culture and Tissues? - Best Practices for Washing Cells After Trypsinization
| Step | Description | Time | Notes |
|---|---|---|---|
| 1 | Remove culture medium and rinse with PBS. | 5 minutes | Use phosphate-buffered saline (PBS) without calcium and magnesium. |
| 2 | Add Trypsin-EDTA solution to cover the cells. | 2-5 minutes | Monitor under a microscope to prevent over-trypsinization. |
| 3 | Stop trypsinization by adding fresh medium. | Immediate | Ensure medium contains serum to inactivate trypsin. |
| 4 | Centrifuge the cell suspension to pellet the cells. | 5-10 minutes | Use a low-speed setting (e.g., 300 x g). |
| 5 | Resuspend the cell pellet in fresh medium. | Immediate | Ensure a homogeneous suspension before plating. |
Handling and Storing Trypsin EDTA for Optimal Performance
Handling and storing Trypsin EDTA is crucial for optimal performance in cell culture. This solution should be stored at -20°C to maintain its efficacy. Avoid repeated freeze-thaw cycles, as they can degrade the enzyme. Use small aliquots to prevent this issue. When you need to thaw it, place it in a 37°C water bath. Always ensure it's fully thawed before use.
Tip: Check for any precipitate before use. If you notice any, gently warm the solution and swirl it to dissolve. This will help maintain its activity in your cell cultures.
For long-term storage, consider using a vacuum-sealed container. This can help keep moisture away and extend shelf life. Always label your containers with dates and concentration. This may seem minor, but it helps avoid confusion later.
Tip: Keep an inventory of your reagents. A simple tracking system can save time and prevent running out unexpectedly. Remember to mix gently before using; aggressive shaking can cause damage to the trypsin. Consistency is key in cell culture techniques for reliable results.